Leading the way to new and better cancer treatments
About Polaris
Polaris Group is a multinational biotechnology company focused on developing novel anti-cancer therapies.
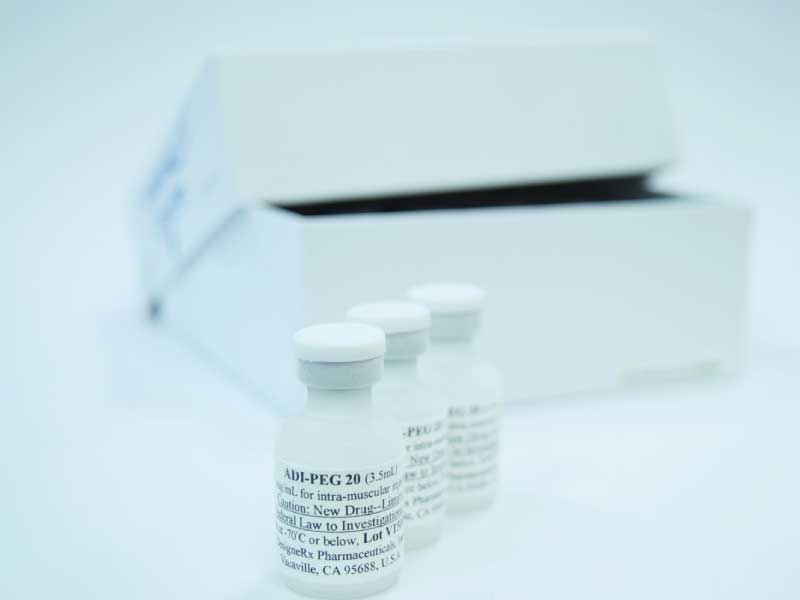
Our lead drug candidate, Pegargiminase (ADI‑PEG 20), is a biologic in late-stage clinical development for a wide range of cancers, including hepatocellular carcinoma, mesothelioma, pancreatic cancer, non-small cell lung cancer, melanoma, acute myeloid leukemia and others.
Polaris Group is involved in every stage of the drug development process. Our family of companies harnesses structure-based drug design technology to create novel oncology therapies, conducts clinical studies at top-tier cancer centers worldwide and operates cGMP Production Facilities in Northern California and China.
Clinical Trials
Pegargiminase is in clinical development for numerous oncology indications.
| Pre-Clinical | Phase 1 | Phase 2 | Phase 3 | BLA |
Mesothelioma
Phase 3 BLA Rolling Submission
Soft Tissue Sarcoma
Phase 3
Glioblastoma
Phase 1/2
Hepatic Cell Carcinoma
Phase 2/3
Acute Myeloid Leukemia
Phase 1
Nonalcoholic Steatohepatitis (NASH)
Phase 2
Development and Manufacturing Capabilities
Company News
English
Polaris Group’s Board Approves Private Placement at a Price Near Market Value
2025/12/29-Polaris Group (TWSE: 6550) convened an extraordinary shareholders’ meeting on the morning of December 26, 2025, at which shareholders approved a proposal for a private ...
Read More
English
Phase 1 Clinical Trial Results of ADI-PEG 20 in Acute Myeloid Leukemia by Polaris Group
Polaris Group (TWSE: 6550) is pleased to announce that its lead drug pegargiminase (ADI-PEG 20) which is already under FDA review for a new drug ...
Read More
English
GENOVIOR, Key Subsidiary of the Company Expands Sterile Injection Capacity to Supply Fully Synthetic Peptides & Specialty Injectables Globally
Genovior Biotech (“Genovior”) announced today the expansion of its sterile injectable manufacturing capacity, enabling large-scale commercial production of fully synthetic peptide medicines and specialty sterile ...
Read More
English
GENOVIOR, Key Subsidiary of the Company and MNR Therapeutics announce an non-exclusive agreement for the commercialization of Fulvestrant in the Gulf Cooperation Council (GCC) region.
2025/11/21-GENOVIOR and MNR Therapeutics announce an non-exclusive agreement for the commercialization of Fulvestrant in the Gulf Cooperation Council (GCC) region.
Read More
Contact Us
Thank you for visiting. Please fill out the form with your specific details and submit it to us. We will contact you shortly. Thank you. *required.


